ocrevus start up form
Ocrevus ocrelizumab fax completed form to 8883021028. The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions.
:max_bytes(150000):strip_icc()/VWH_Illustration_Drugs_Ocrevus-Ocrelizumab_Illustrator_Zoe-Hansen_Final-ebd309090cd04981957356e384d5f251.jpg)
Ocrevus Ocrelizumab Intravenous Uses Side Effects
OCREVUS was not associated with.

. 300 milligrams mg per 10 milliliters mL of solution. OCREVUS is a prescription medicine used to treat. Before starting treatment with Ocrevus your doctor will explain your dosing schedule.
Prescription Enrollment Form. Take a photo and text it to 650 877-1111 Print. Ocrevus ocrelizumab Fax completed form to 8883021028.
Genentech can start supporting you when PAGE 4 of this form is submitted by you or your doctors office in one of the following ways. See full safety for more information. Submit Print Or Download Ocrevus Forms Documents Ocrevus Access Solutions Genentech Ocrevus Ocrelizumab Multiple Sclerosis Disease Modifying Therapies The.
Find Information DMTdisease-modifying therapy. Ocrevus is indicated for the treatment of. Ocrevus is a prescription medicine used to treat.
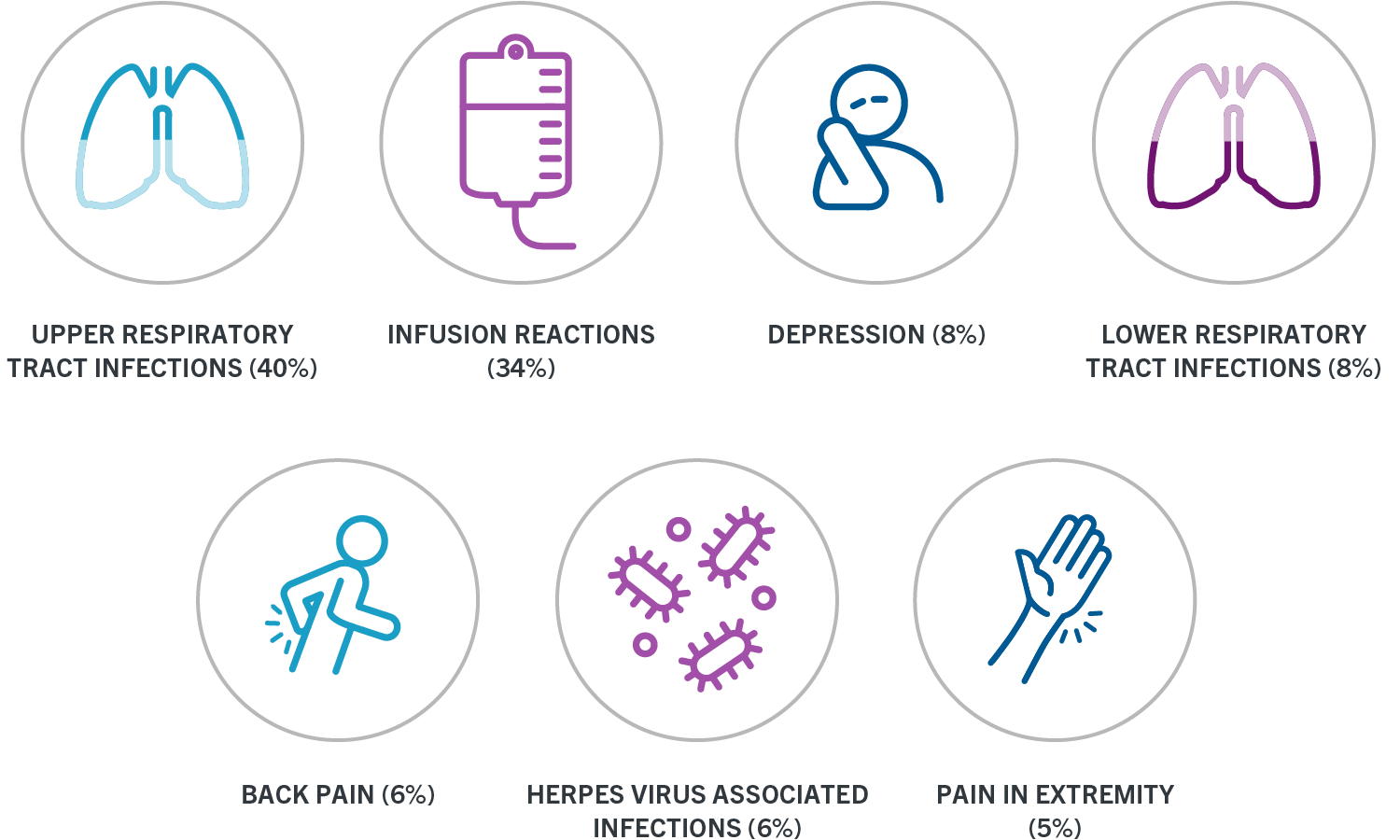
Ocrevus comes in one strength. Sign a printed form and fax or mail it to us or give it to your doctors office to do so Your doctor also has to fill out a form called the OCREVUS Start Form. OCREVUS increased the risk for upper respiratory tract infections lower respiratory tract infections skin infections and herpes-related infections.
Primary progressive ms in. OCREVUS increased the risk for upper respiratory tract infections lower respiratory tract infections skin infections and herpes-related infections. Date of birth Prescribers first name.
It is a one-time registration completed by the practice. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary. The form includes patient insurance and prescription information.
Talk to your healthcare provider about registering with the OCREVUS Pregnancy Registry. Genentech can start supporting you when PAGE 4 of this form is submitted by you or your doctors office in one of the following ways. It must be completed by the provider.
OCREVUS was not associated with. 650 877-1111 Call Visit Online Fax Genentech. This form is used to initiate the EFT registration process when the practice chooses not to use check reimbursements.
OCREVUS increased the risk for upper respiratory tract infections lower respiratory tract infections skin infections and herpes-related infections. OCREVUS was not associated with. Once we have both these forms.
Typical dosage Typically your doctor will recommend that you take a starting dose of. Learn about OCREVUS ocrelizumab a prescription medicine used to treat adults with relapsing or primary progressive multiple sclerosis. OCREVUS was not associated with.
OCREVUS increased the risk for upper respiratory tract infections lower respiratory tract infections skin infections and herpes-related infections. The purpose of this registry is to collect information about your health and your babys health. Access the OCREVUS Start Form and learn more about the assistance Genentech offers for your OCREVUS ocrelizumab patients.
Take a photo and text it to 650 877-1111 Print. The initial dose of Ocrevus will be 300 mg given over 25 hours or more.
:max_bytes(150000):strip_icc()/md-vs-ms-5121186.FINAL-8585a0075b2745a187afca64357afc9c.gif)
Md Vs Ms Muscular Dystrophy And Multiple Sclerosis

Most Impressive Drug Launch Roche S Ocrevus Biopharma Dive
A Resilience Group Training Program For People With Multiple Sclerosis Results Of A Pilot Single Blind Randomized Controlled Trial And Nested Qualitative Study Plos One
Benefits Investigations Prior Authorization Resources Ocrevus Access Solutions

Home Page Multiple Sclerosis And Related Disorders
Frontiers Multiple Sclerosis And Cancer The Ying Yang Effect Of Disease Modifying Therapies

Ectrims2022 Targeting Ebv Key In Progressive Ms Experts Ata188 May Be Game Changing Treatment For Non Active Forms Multiple Sclerosis News Today

Multiple Sclerosis A New Treatment A New Hope Pacific Neuroscience Institute
:max_bytes(150000):strip_icc()/types-of-multiple-sclerosis-5200710_final-80ec48e459554962a559890ad290a980.jpg)
Types Of Multiple Sclerosis Ms Progression Outlook

Safety Tolerability And Activity Of Mesenchymal Stem Cells Versus Placebo In Multiple Sclerosis Mesems A Phase 2 Randomised Double Blind Crossover Trial The Lancet Neurology

Multiple Sclerosis Symptoms And Causes Mayo Clinic

Submit Print Or Download Ocrevus Forms Documents Ocrevus Access Solutions

Ocrevus Start Form Pdf Fill Online Printable Fillable Blank Pdffiller

Ms Clinic Referral Form Fill Out Sign Online Dochub

Ms Progression Chart Stages Of Ms Disability Scale And More

Ocrevus After One Year On The Market What Do We Know Now Everyday Health